Key Points:
- In today’s Recommendation for Industry, we discuss the “why” behind testing at Day 6 if you’ve been exposed and testing multiple times for COVID if you have symptoms. Read more below.
- CDC unveils streamlined COVID-19 guidance. As was mentioned last week, the expected guidance update was published on Thursday. Key aspects include recommendations to stay up to date with vaccinations. If you’ve been exposed, mask for 10 days and get tested on Day 6. Isolate if you test positive or have symptoms but don’t yet have test results. If positive, stay home and isolate for 5 days; then, if fever-free for 24 hours and symptoms improving, can end isolation. Mask through Day 10. If symptoms worsen after isolation ended, restart at Day 0.
- FDA: Multiple at-home COVID tests needed to confirm negative result. The FDA recommended that people take multiple at-home COVID-19 antigen tests to confirm a negative result, whether they have symptoms or not. The recommendation includes the use of multiple tests over a certain time period, such as 2 to 3 days, then at least one extra test taken 48 hours later to confirm a negative result in people with symptoms. Those without symptoms who think they were exposed should take at least two additional tests, at 48-hour intervals, after a negative result.
- WHO unveils new monkeypox variant names. As the first step in renaming the monkeypox virus and its variants, an expert group convened by the World Health Organization (WHO) agreed on new names for the variants, as the WHO continues collecting proposals on a new name for the disease itself. The experts reached consensus on names for the clades: clade 1 for the former Congo basin (Central African) clade and clade 2 for the former West African clade. The two clade 2 subclades will be known as clade 2a and clade 2b. The clade 2 variants are the main ones circulating in the current global outbreak.
Food Safety & Public Health
- Polio found in NYC wastewater; local circulation likely. Sequencing tests suggest the virus in New York City wastewater is either vaccine-derived poliovirus or variants of the revertant polio Sabin-like type 2 poliovirus, both of which can cause illness in people. CDC stated that there is no cure for polio, but it is preventable through safe and effective vaccination. People most at risk for infection are those who never had the polio vaccine; those who never received all the recommended vaccine doses; and those traveling to areas that could put them at risk for getting polio.
- FDA Releases Paper-Based Versions of the Agricultural Water Assessment Builder Tool. The FDA has released paper-based versions of its agricultural water assessment builder tool in both English and Spanish. The assessment builder was released in March as an online tool to help farms understand the proposed requirements in the Agricultural Water Proposed Rule. The paper-based version is intended to make the content more accessible to a broader array of users. Use is optional.
- FDA – Rumor Control. Earlier this month, FDA published a paper on “Rumor Control.” Stating that “the growing spread of rumors, misinformation and disinformation about science, medicine, and the FDA, is putting patients and consumers at risk,” the paper includes COVID-19 FACTS Q&A. FDA Commissioner Robert Califf has said that combating misinformation is one of his priorities.
- August is National Immunization Month. National Immunization Awareness Month (NIAM) is an annual observance held in August to highlight the importance of vaccination for people of all ages.
Recommendations for Industry
The “Why” Behind Day 6 and Multiple COVID Testing
The updated CDC guidance recommends that those who are exposed to COVID start precautions immediately by masking, then test on day 6. Meanwhile FDA has added recommendations for multiple testing over two to three days and one to two tests 48 hours later if results are negative. Why are these recommendations being made?
As the BMJ graphic below shows, the viral load of COVID is negligible immediately after exposure, then begins to build in successive days, with the highest viral load – and generally the beginning of symptoms – showing at 6 days after exposure. Thus, it is at this time that the test is likely to be the most accurate.
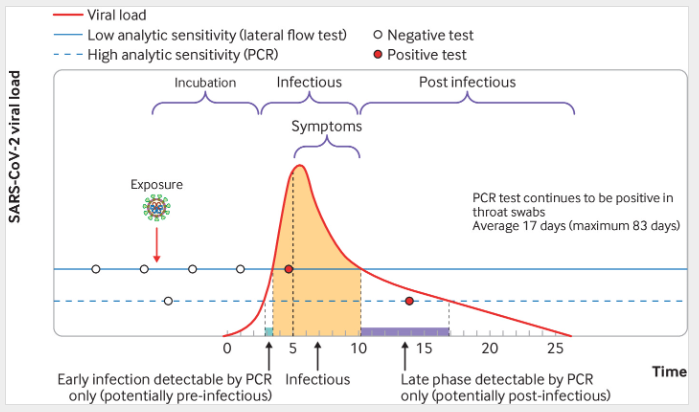
A number of other factors also can lead to false positives, particularly with home test kits, including inaccurate testing (swab not placed deep enough or left long enough, etc.). The vaccination or previous infection history of a person also can impact time to positive, with anecdotal evidence of increased number of boosters or previous infection delaying the time to positive from exposure. Immunologically, this makes sense as someone with prior immunity will mount an immune response (and experience symptoms), which may suppress the viral load early in the course of infection.
Multiple tests also are recommended due to the higher limit of detection (more virus is needed to trigger a positive result) of the rapid antigen tests (vs. PCR). As found in a Cochrane study, antigen tests correctly identified COVID-19 infection in about 73% of people with symptoms and only 55% of people without symptoms. Thus, while the at-home tests provide a fast, convenient way to test, repeated tests, particularly with negative readings, will provide the most accurate results.
With all this, TAG continues to recommend that anyone with symptoms, regardless of the source (COVID, flu, etc.), stay home so as to lessen the potential transmission of any communicable disease.
In case you missed it:
- In last Thursday’s Recommendation for Industry, we discussed the positive COVID trends we are seeing. Read more here.
- Study: Pfizer COVID vaccine efficacy wanes 27 days after dose 2 in teens. A new study finds waning Pfizer/BioNTech COVID-19 vaccine protection against symptomatic infection in Brazilian and Scottish teens starting 27 days after the second dose amid the Delta and Omicron variant waves, but protection against severe illness was still strong at 98 days in Brazil. During Omicron, 27 days after the second vaccine dose, VE against symptomatic infection began to fall, plummeting to 5.9% (95% CI, 2.2% to 9.4%) in Brazil and 50.6% (95% CI, 42.7% to 57.4%) in Scotland at 98 days or more. Over the same period after dose two in Brazil, VE against severe infection stayed above 80% at 28 days and was 82.7% (95% CI, 68.8% to 90.4%) at 98 days or more. The researchers noted that two doses of vaccination with BNT162b2 [Pfizer] among adolescents are insufficient to sustain protection against symptomatic disease; however, they do offer substantial protection against serious COVID-19 outcomes for at least 3 months.
- Omicron subvariants gain more ground, including BA.4.6 in the Midwest. Though the 7-day average for new daily COVID-19 cases is slowly declining, the more transmissible and immune-evasive Omicron subvariants became even more dominant last week, with an offshoot called BA.4.6 gaining traction in some Midwestern states. In its weekly variant proportion updates today, the Centers for Disease Control and Prevention (CDC) said the proportion of BA.5 viruses in sequenced samples last week rose from 84.5% to 87.1%, while BA.4 declined slightly, from 8.2% to 6.6%. However, the proportion of BA.4.6 viruses rose from 4.2% last week to 4.8% this week. Midwestern states—Iowa, Kansas, Missouri, and Nebraska—are seeing the highest BA.4.6 proportions, where it makes up 13.2% of sequenced specimens.
- Global COVID cases level, deaths decline. After a period of declining cases, global COVID-19 cases have begun to stabilize. There are still cases increasing in Japan and South Korea. Deaths declined 9% last week compared to the previous week, with about 14,000 fatalities reported, the WHO said. In the United States, the 7-day average for new daily cases is 108,820, according to a Washington Post analysis. The 7-day average daily death count isn’t declining as the case rate is within the US, these numbers sit at 499.
Public Health & Food Safety:
- Monkeypox:
- US to begin intradermal injections of Jynneos, stretching the supply. Stretching the supply of Jynneos will allow clinicians to use one-fifth the amount of vaccine per patient to expand the limited supply within the United States. The United States had made 1.1 million doses of Jynneos available and about 600,000 doses have already been distributed. The remaining doses, under the new intradermal rule, will turn into just under 2 million doses. The CDC’s current recommendation is to vaccinate close contacts and those at risk for contracting the virus, including those who have had multiple sexual partners in the last 14 days in an area with high monkeypox transmission. Evidence that the technique is effective is limited to one study conducted in 2015, in which two intradermal doses produced similar results to subcutaneous injections. Those who may have already received their first dose as subcutaneous can receive their second dose as intradermally. The nation’s total has raised to 8,934 cases. As Montana just discovered its first case, Wyoming is now the only state without a recorded case. Global counts sit at 31,000 cases in 93 countries.
- Following the discovery of type 2 vaccine-derived poliovirus in sewage in north and east London, the Joint Committee on Vaccination and Immunisation (JCVI) has advised that a targeted inactivated polio vaccine (IPV) booster dose should be offered to all children between the ages of 1 and 9 in all London boroughs.
- USDA gets employee safety training and OSHA gets access to food facilities under new MOU. FSIS and OSHA will coordinate FSIS efforts with workplace hazards and conditions training. The training must be completed within 120 days, with annual refresher training. At FSIS-regulated establishments, OSHA-provided posters will be made available on how to report injuries to OSHA. The agencies have cooperated for almost 30 years to protect workers, signing their first Memorandum of Understanding (MOU) for employee safety on Feb. 4, 1994.
- Cyclospora patient count grows in outbreak of unknown origin. The Food and Drug Administration says there are now 77 confirmed patients compared with 60 a week ago. The agency has not yet determined what food is the source of the parasite. There is a steady increase in individuals obtaining this illness. The FDA also reports that it has begun traceback efforts in relation to a separate outbreak of Cyclospora infections, but the agency has not revealed what food or foods are being traced.





