If you’re returning to the U.S. on an international flight and get asked if you’d be willing to volunteer to take a COVID test, it’s likely part of CDC’s Traveler-based Genomic Surveillance program (TGS). With U.S. airports visited by more than 1 billion travelers each year, and travelers being a significant mode for the spread of pathogens and emerging infectious diseases, CDC sees strategic biosurveillance at airports as one means of detecting of SARS-CoV-2 variants and other pathogens of public health importance in a timely manner.
With a goal of early detection and filling in gaps in global biosurveillance, the nasal sampling multi-pathogen pilot was recently expanded to test for Flu A/B, RSV, and other respiratory pathogens, along with SARS-CoV-2. Currently in operation at six major US international airports: Los Angeles, Newark, Seattle, Washington, DC (IAD), Boston, San Francisco, and NYC (JFK), it is being implemented by Ginkgo Bioworks and XpresCheck and scheduled to last for several months.
Here’s how it works:
- International travelers arriving at participating airports are asked to volunteer.
- Participants self-collect nasal swab samples and answer a short survey.
- The samples, which are deidentified, are shipped to a laboratory network for testing.
- Positive samples undergo whole genome sequencing to determine variants.
- Select TGS samples are shared with CDC’s laboratory where they undergo viral characterization for information about a new variant’s transmissibility, virulence, and response to current treatments or vaccines.
As of September 2023, TGS has enrolled more than 360,000 air travelers. Participation in the program is voluntary and anonymous. The program covers flights from more than 135 countries from all World Health Organization regions.
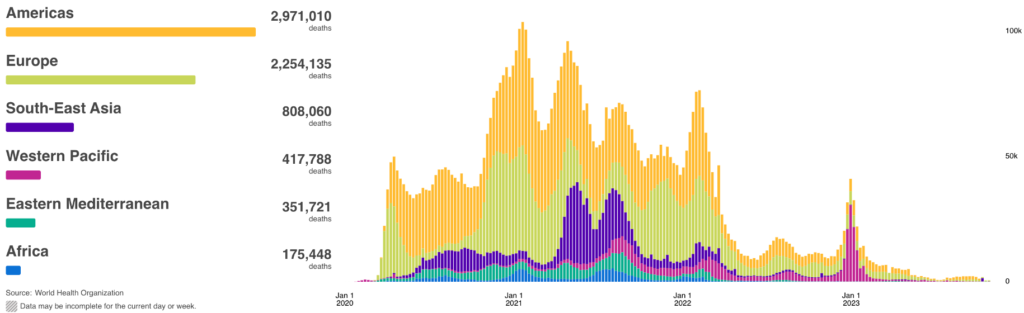
COVID Risk Matrix:
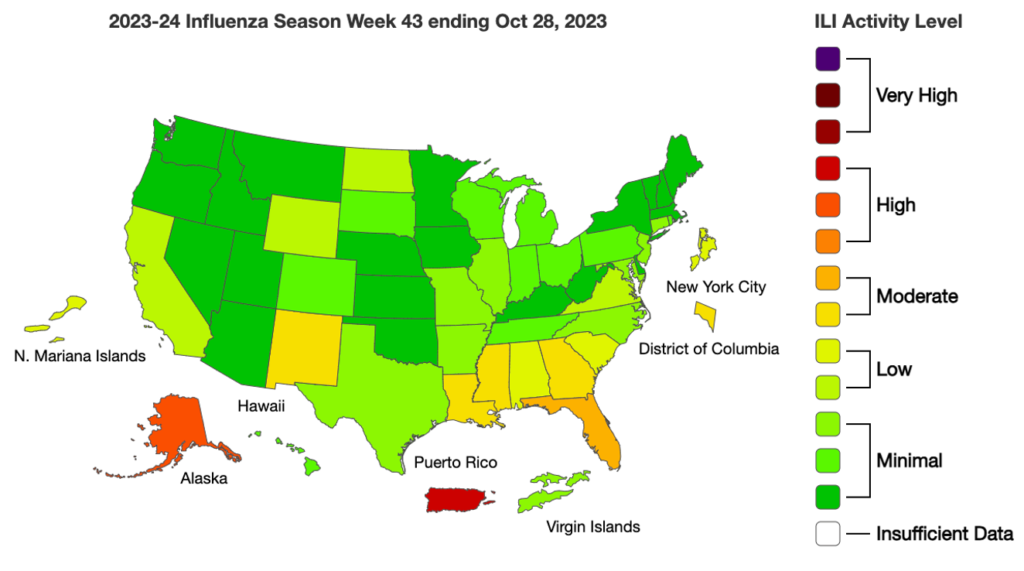
Influenza:
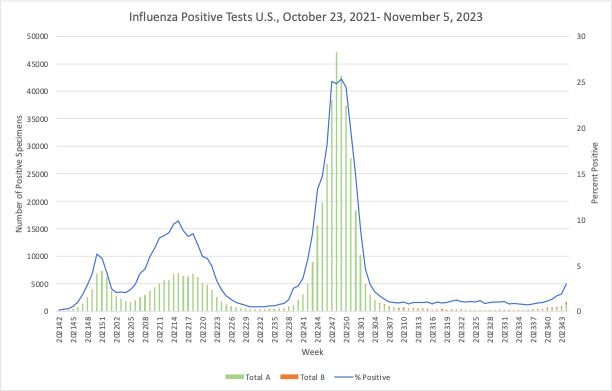
Infectious Disease News:
- Coadministration of the BNT162b2 BA.4/5 bivalent mRNA COVID-19 vaccine and seasonal influenza vaccine generally has similar effectiveness against COVID-19- and SIV-related outcomes compared with administration of each vaccine alone.
- In a high proportion of meningitis cases, the causative pathogen remains unknown despite extensive diagnostic testing. A study investigated samples from patients hospitalized in southern Spain in 2015–2019 with undetermined cause of aseptic meningitis. Toscana virus (TOSV), a virus transmitted by sandflies, was identified in eight of 23 samples from patients with meningitis of unknown origin. Of the eight TOSV-positive patients, three had occurred in autumn and five lived in urban areas. Only one recalled recent insect bites, an event that may raise suspicion of TOSV infection.
- A case of active TB in a young child has been confirmed at a drop-in day care center at a YMCA in West Omaha. Up to 500 children and staff may have been exposed from late May through Oct 24th and contract tracing is underway. TB can be especially serious in very young children.
- The FDA approved the first vaccine to prevent disease caused by chikungunya virus. The vaccine is approved for individuals 18 years of age and older who are at increased risk of exposure. This virus is primarily transmitted to people through the bite of an infected mosquito. There have been at least 15 million cases reported globally in the last 15 years.
- The FDA cleared the first Covid-19 home antigen test. ACON Laboratories’ Flowflex Covid-19 Antigen Test was originally authorized for emergency use in 2021 but is now the second Covid home test to successfully complete a traditional FDA premarket review. The test can be used on individuals 2 years of age and older.
- As of October 14, data from a survey from the CDC revealed that just over 7 percent of adults and 2 percent of children had received the latest Covid-19 vaccine.





