When an outbreak appears to be caused by an FDA-regulated human food, a team from the agency’s Coordinated Outbreak Response & Evaluation (CORE) Network takes on the charge to coordinate FDA response efforts and review data to aid in the understanding of emerging outbreaks. As evidenced by CORE’s most recent annual report, there is a lot that can be learned from previous incidents for future prevention.
CORE was established in 2011, in response (in part) to the 2006 E. coli spinach outbreak, for which FDA advised consumers to avoid eating any fresh spinach prior to the outbreak’s linkage with a specific brand. The economic harm of this massive avoidance was one of the triggers mentioned in the GAO report, FDA’s Food Advisory and Recall Process Needs Strengthening, for the founding of CORE. While there continue to be distinct opportunities for improvement in the way and speed at which recalls are handled, prior to CORE, FDA responded to outbreak events “by bringing staff together on an ad hoc basis.” Thus, the coordinated focus of CORE and, particularly, its focus on data review from food firms (e.g., past inspections, sampling results, product distribution, sourcing information, and previous incidents involving similar pathogen and food pairs) is contributing to a deeper understanding of causes, source identification, and (for better or worse) enforcement action.
In early January, CORE released its annual report, which summarizes adverse events and outbreaks linked to FDA-regulated foods it investigated in 2022. Overall, CORE evaluated 65 incidents, for which 28 responses were initiated, and 11 advisories issued. Additionally,
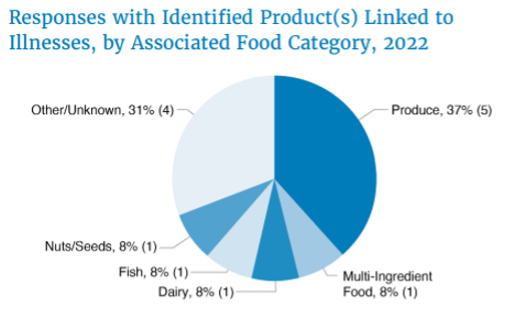
- Of the 13 outbreaks passed to the CORE response team, more than a third related to produce.
- In addition to produce, the identified product categories were dairy, fish, nuts/seeds, and multi-ingredient, along with four outbreaks of other or unknown origin.
- New adverse trends investigated included those related to infant formula, a meal replacement drink, dry cereal, and a frozen food product.
- Described as noteworthy were outbreaks related to enoki mushrooms, cantaloupe, and peanut butter.
- The 11 advisories issue for multistate outbreaks of foodborne illnesses or adverse events were associated with nine product recalls.
- Subsequent enforcement actions included warning letters, import alerts, and a consent decree
Investigations of new adverse trends, which in 2022 included infant formula, a meal replacement drink, dry cereal, and a frozen food product, have unique challenges in that they rely on self-reported complaints from consumers and healthcare providers and voluntarily submitted data from industry. Thus, the investigators may not always receive all the information needed to fully investigate the product or event. Additionally, laboratory test results that are generally available in an outbreak investigation are not always available. Despite that, such investigations can, and do, result in enforcement action, such as the consent decree of permanent injunction, which required the infant formula manufacturer “take the steps necessary to safely produce infant formula in close coordination with FDA and under our oversight of its manufacturing and food safety processes.”
Following are some the four 2022 recalls that CORE found to be noteworthy:
- The linkage of Listeria-contaminated Enoki mushrooms imported from China was linked to a previous 2020 outbreak of enoki mushrooms from the Republic of Korea in 2020, the second from product imported from China in 2022. With the 2020 outbreak prompting the development of a Strategy to Help Prevent Listeriosis and Salmonellosis Outbreaks Associated with Imported Enoki and Imported Wood Ear Mushrooms, more than 25 recalls of enoki mushrooms have been conducted, and the initial import alert for the Korean mushrooms was expanded to those from China.
- In the outbreak investigation of Salmonella Typhimurium-contaminated cantaloupe, the FDA, CDC, and state partners all worked together. Based on traceback information, FDA conducted investigations in Indiana at three farms, their common packinghouse, and nearby public lands. Although Salmonella positive environmental samples were found at each location, none of the isolates conclusively matched the outbreak strain by whole genome sequencing (WGS). Because the outbreak vehicle was not confirmed until after the outbreak ended and product was no longer on the market, no recall or no public warning was issued.
- The multistate outbreak of Salmonella Senftenberg linked to peanut butter products investigated by he FDA, CDC, and state and local partners, led to a voluntarily recall for the implicated products, and a warning letter was issued as a result of an inspection conducted in the investigation. FDA is currently preparing communications to discuss findings and provide information to assist in future prevention efforts.
While it can be hoped that any outbreak (or recall or close call) provides for lessons learned and preventive action against future incidents, outbreaks such as the above are exemplary in their evidence of continually emerging trends and food-pathogen pairs. While a risk-based approach appropriately prioritize foods and contaminants that have historically caused the greatest issues, emerging incidents show that it is not sufficient to focus only on the highest risk foods. Foods producers must continually stay informed of industry trends and emerging risk, both from your own sources (e.g., customer complaints) as well as outbreaks and recalls across your industry segment and the food industry as a whole.
The CORE Network publishes a publicly available Investigation Table that is updated weekly and provides information about foodborne illness outbreak and adverse event investigations. Additionally, FDA’s Warning Letters webpage can keep you informed of FDA-cited noncompliances, root causes, and appropriate (and non-appropriate) responses. TAG has experts in all facets of food safety, and we periodically review current Warning Letters for key failures and recommendations. To stay up to date, subscribe to the TAG newsletters. And give us a call should you need any assistance.