Based on the current and expected evolution of SARS-CoV-2, the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC) is advising vaccine manufacturers to update formulations to be used beginning this fall to the monovalent XBB.1.5 lineage of the Omicron variant.
The unanimous decision for the XBB lineage was made during the committee’s June 15 meeting based on both manufacturing timelines and available data including that of the current circulation of virus variants and current vaccine effectiveness. Other evidence influencing strain selection included virus surveillance and genomic analyses, antigenic characterization of viruses, human serology studies from current vaccines, pre-clinical immunogenicity studies evaluating immune responses generated by candidate vaccines.
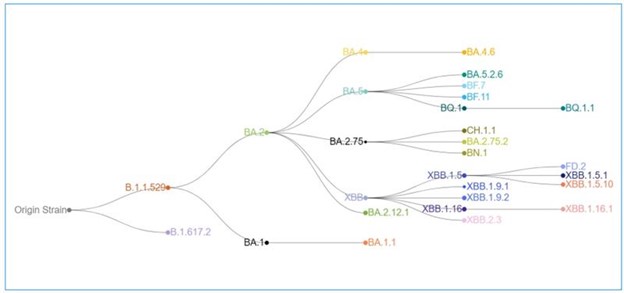
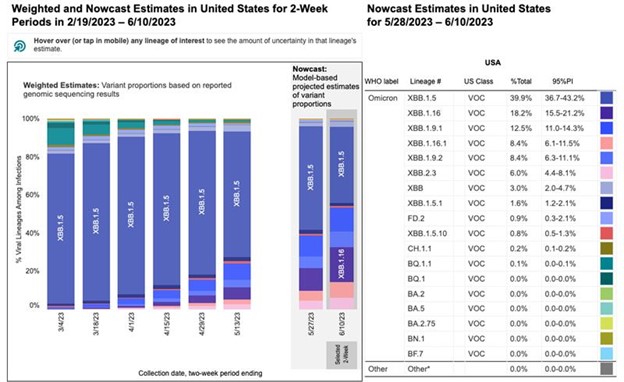
Data on COVID evolution indicates that the XBB lineages accounted for more than 95% of the circulating virus variants in the U.S. as of early June 2023. While XBB.1.5 has declined to less than 40% of presumed circulating virus in the U.S., XBB.1.16 is on the rise and XBB.2.3 is slowly increasing in proportion (CDC COVID Data Tracker: Variant Proportions).
The COVID evolution and current variant estimates are depicted in the following graphics.
Influenza:
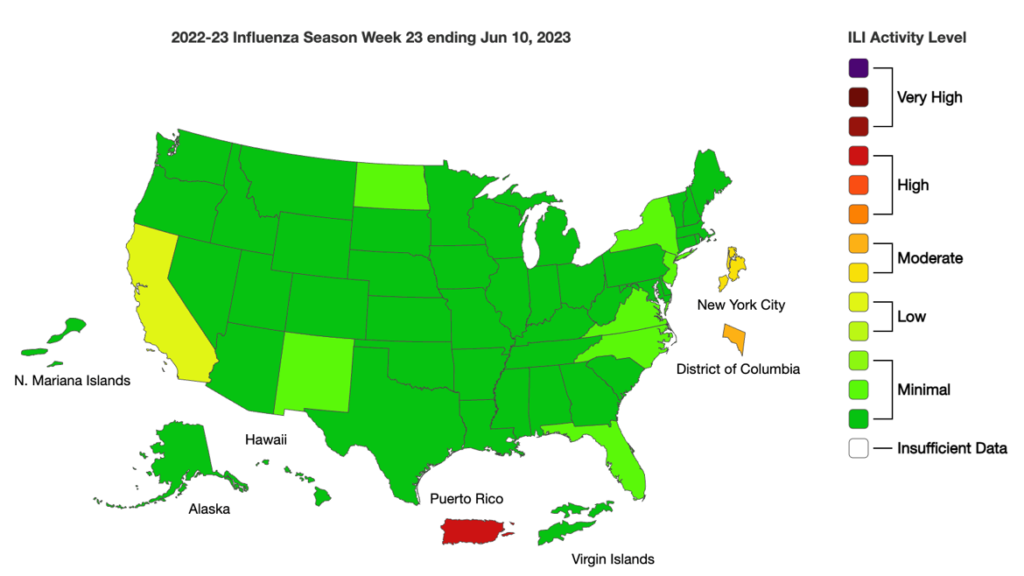
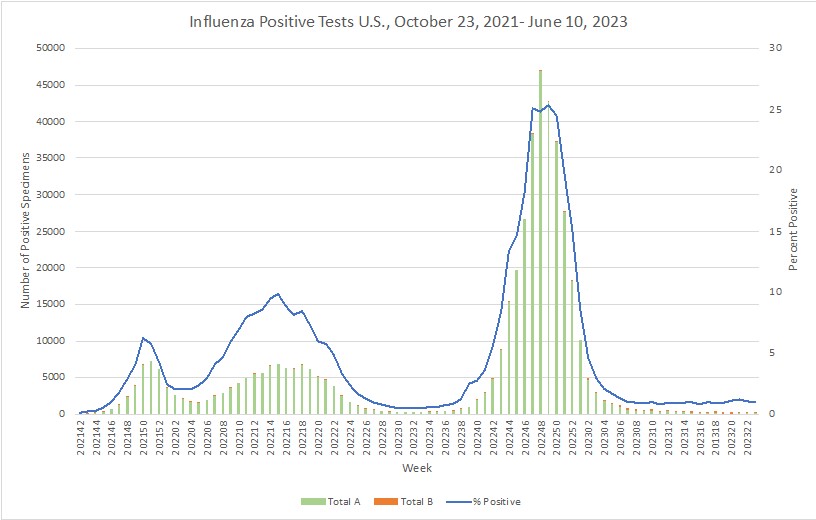
- Although flu cases in Australia quickly rose this season, they are still trending lower than in 2022 at this time.
- From the beginning of the year through May 7, 2023, nearly 100,000 suspected measles cases and 1,239 deaths were reported from 459 health zones across the Democratic Republic of the Congo, resulting in a case fatality rate of 1.2%. This is an almost 100% increase in cases. A measles outbreak is ongoing in Austria, with 121 cases reported as of 13 June 2023. The number of cases is currently decreasing.
- A norovirus outbreak in Hawaii in May has been definitively linked to the consumption of raw oysters from South Korea based on FDA’s recent tests that detected GII norovirus in oyster samples. The MN state lab also detected the same virus. Previous warnings to not consume these oysters have been issued.
- The annual Hajj pilgrimage, which usually attracts on average two million people, will take place between June 26 and July 1 in Saudi Arabia, open to anyone at least 12 years old. Participants are provided with a range of requirements and standard recommendations, including vaccination requirements for COVID-19, meningococcal infection, and depending on where they come from, poliomyelitis and Yellow fever. Returning travelers from the Hajj should seek medical attention immediately if they experience symptoms suggestive of any type of infection.
- The FDA has recommended XBB.1.5 for the fall monovalent COVID vaccines.