While COVID numbers remain comparably low, there are some indications that we might see an uptick in cases by the end of the summer. This is due, in part, to the high levels of travel – as TAG has seen itself with about 15% of our own workforce contracting COVID over the last two months, with some fairly severe symptoms. Additionally, CDC statistics are showing a slow uptick in hospitalizations due to COVID, with a 12.1% increase over the week of July 16 to 22, and FDA has extended its enforcement discretion policies for supplier onsite audits.
From all this, we are seeing not only a current rise, but also see it as likely that there will be a surge of illness in the fall and early winter as school resumes, people stay indoors, and holiday travel picks up.
So, what does this mean for businesses, and what should you be doing?
COVID’s biology hasn’t really changed, and people are most infectious in the 5-day period between symptom onset and improvement in symptoms. So the CDC (and TAG) still recommends that people stay out from work for 5 days after symptom onset if they’re diagnosed with COVID, and that they wear a mask between day 6 and 10 if they’re well enough to return to work.
For businesses that are able to do so, providing COVID pay to encourage employees to stay out and not infect coworkers is the most protective approach. Understanding that this is not viable for all, maintaining wellness checks and reminding employees to stay home when ill remain important for the health of your workforce. TAG also encourages facilities to think about air filtration, such as using HEPA filtration units in conference rooms and using MERV 13 filters wherever possible.
With the FDA extending the duration of enforcement discretion policies relative to foreign supplier onsite visits for the FSMA Preventive Controls, FSVP, and Accredited Third-Party Certification rules, it is also essential that businesses maintain and monitor high supplier requirements themselves. Not only does this help protect your product and business, but once FDA withdraws the extended guidance and resumes its visits, inspectors are likely to be intense in their inspections, quickly citing any fall-off in food safety compliance.
COVID Risk Matrix:
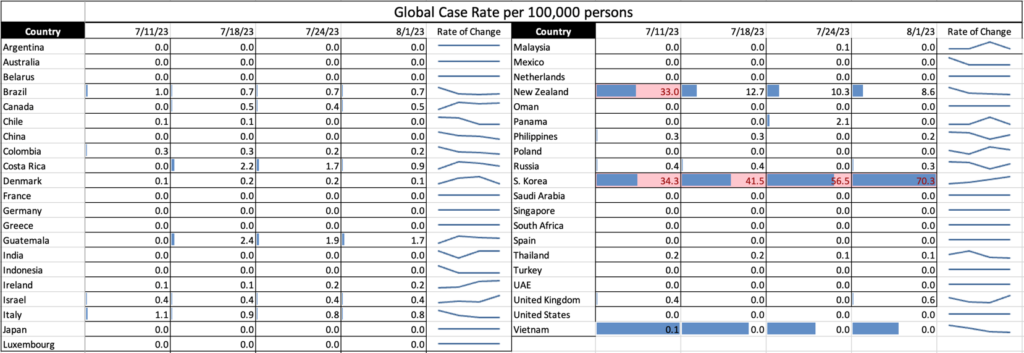
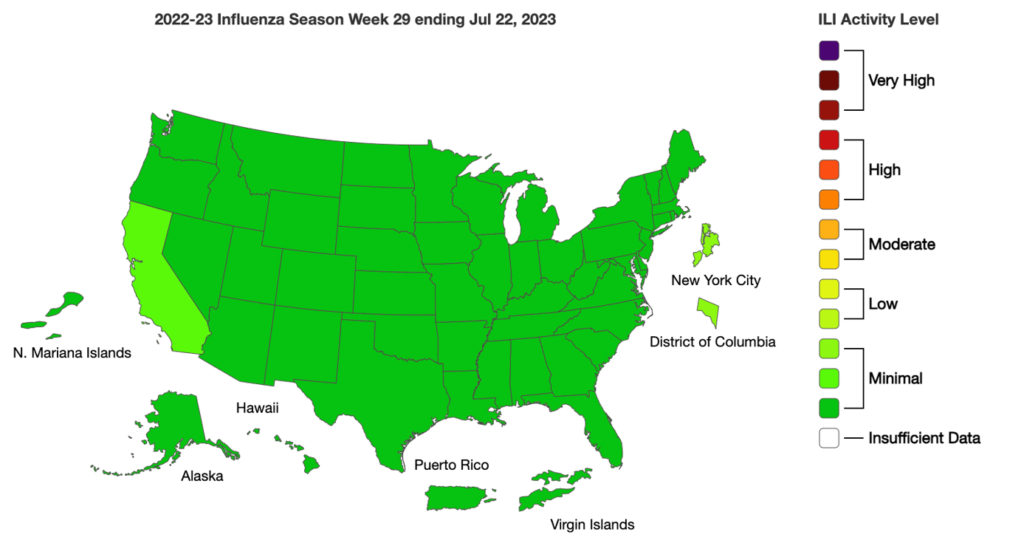
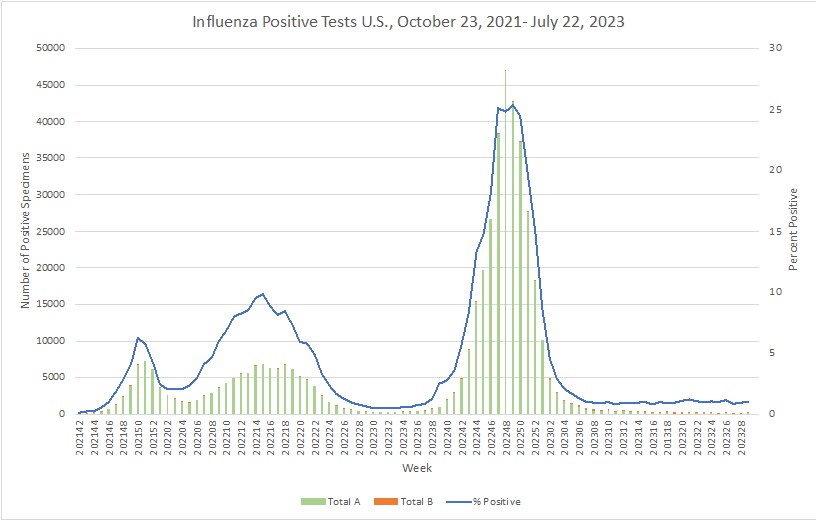
Influenza:
- The FDA approved Ervebo, a vaccine for the prevention of Ebola for individuals 12 months through 17 years of age. This vaccine has been approved for individuals 18 and older since December 2019.
- Cases of measles in children are up across Europe. It is estimated that vaccination rates are only at about 89%.
- From June 21, 2023 to July 20, 2023, 57,024 new cholera cases, including 399 new deaths, were reported worldwide.
- Although the COVID-19 activity throughout the US is still very low, markers that health officials use to track illness activity has shown small rises for the third week in a row, according to the CDC. WHO has reported that very few countries are reporting rises in hospitalizations and intensive care unit admissions.





