TAG Expands Assessments to Include Current Communicable Disease Data
Key Points:
COVID
- In today’s Recommendation for Industry, we discuss TAG expanding our public health offerings to include focus on other workplace communicable diseases. Read more below.
- Global COVID-19 cases down 24%. The WHO’s weekly epidemiologic update showed official worldwide reports of 5.4 million new COVID-19 cases last week, down 24% from the week before, and 15,000 deaths, down 6%.
- Updated COVID boosters could be available in 3 weeks, White House predicts. Newly updated COVID-19 booster will be available in the next three weeks or so, if the FDA and CDC work through their processes for authorization as expected. In late June, the FDA directed Moderna and Pfizer to make vaccines that targeted the more contagious BA.5 Omicron subvariant and the original COVID strain. That work has been underway, and the next step is for the FDA and CDC to receive and review data from the companies. Neither has announced a timeline.
- Study: 56% of Omicron-infected adults didn’t know they were contagious. An observational study of 210 adults in California with detectable SARS-CoV-2 antibodies during an Omicron variant wave shows that 56% didn’t know they had been infected, with some attributing symptoms to a cold or other non-COVID infection. The JAMA Network Open report finding fuels concerns about asymptomatic transmission, particularly by those with lesser general health awareness and those without daily screening protocols supported by COVID-19 sick pay policies.
- Study shows potential benefits of mix-and-match approach for COVID-19 boosters. A “mix-and-match” strategy for COVID-19 vaccine boosters may help improve the durability of antibody and T-cell responses against the Omicron variant, according to a Harvard Medical School study. The study found that people who received a booster dose of the J&J adenovirus vector vaccine after primary immunization with the Pfizer messenger RNA vaccine had durable responses for at least 4 months.
Monkeypox
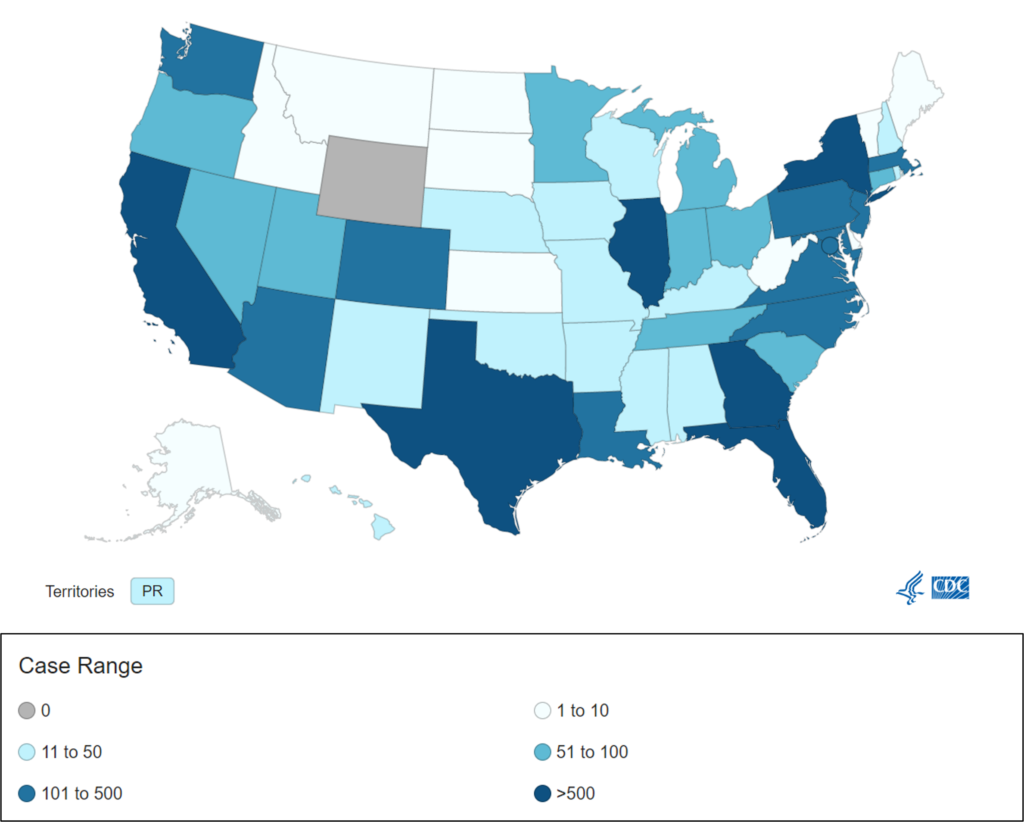
- WHO: Monkeypox cases rose 20% in past week. According to the World Health Organization (WHO) monkeypox cases increased by about 20% for the second consecutive week, with most case increases seen in Europe and North America. More than 35,000 cases have been reported from 92 countries and territories, with 12 deaths. Almost all cases continue to be reported among men who have sex with men. In the U.S., CDC reported 799 more cases, raising the total to 12,689. New York has the most cases (2,620) followed by California (1,945).
- Studies heighten concerns about asymptomatic monkeypox spread. In one study, lab tests from asymptomatic French men who were sampled regularly for other purposes were positive for monkeypox. The authors said it’s not clear if viral shedding can lead to transmission. Another case study showed that a man sought care for a week-long vesicular rash he developed after a trip to the UK, where he attended a large, crowded outdoor event that involved close contact, including dancing; his sharing of an e-cigarette with a woman he met; and many attendees wearing shorts and sleeveless tops. The studies suggest that vaccination of only known contacts may not prevent spread; there may be potential for spread at large events; and fomites may be another potential transmission mode.
Food Safety & Public Health
- CDC director calls for agency changes following pandemic missteps. In an email to employees, CDC Director Dr. Rochelle Walensky said the agency must make drastic changes to respond better and faster to public health emergencies, following missteps during the COVID pandemic. With a goal of “a new, public health action-oriented culture at CDC that emphasizes accountability, collaboration, communication, and timeliness,” she wrote, the agency needs to overhaul how it analyzes, shares, and communicates data – sharing data faster and in a way that speaks to the American public in easy-to-understand language. It’s also anticipated that leadership changes and internal reorganizations will occur.
- New Online Toolkit to Help Small Processors Comply With FSMA. The Northeast Center to Advance Food Safety (NECAFS), a University of Vermont Extension program, published a new webpage that features a food safety toolkit tailored to small and very small processors that are required to comply with FSMA’s Preventive Controls Rule.
- Salmonella linked to backyard poultry sickens 312 more people. In its latest update on the Salmonella outbreak linked to backyard poultry, the CDC reported 312 more cases, raising the nation’s total to 884 in 48 states and the District of Columbia. The five states with the most cases are California (59), Minnesota (46), Texas (45), Wisconsin (41), and Missouri (36). Two more Salmonella subtypes were added to the outbreak.
- C.D.C. Investigates ‘Fast-Moving’ E. Coli Outbreak. Federal health officials are investigating an E. coli outbreak that has sickened 14 people in Ohio and 15 in Michigan, with nine people hospitalized and no reported deaths. The CDC said that no food had been identified yet as the source, but laboratory tests have shown that the patients are infected with the same type of E. coli.
Recommendations for Industry
TAG Expands Assessments to Include Current Communicable Disease Data
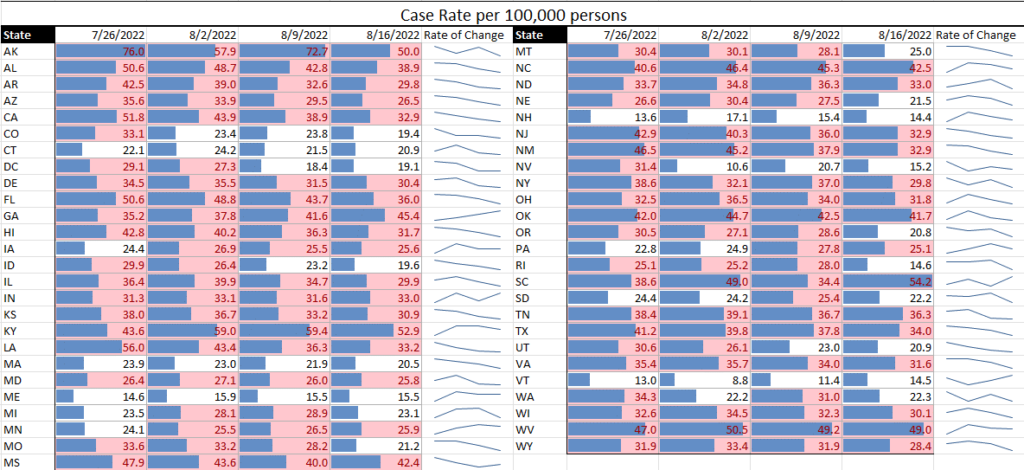
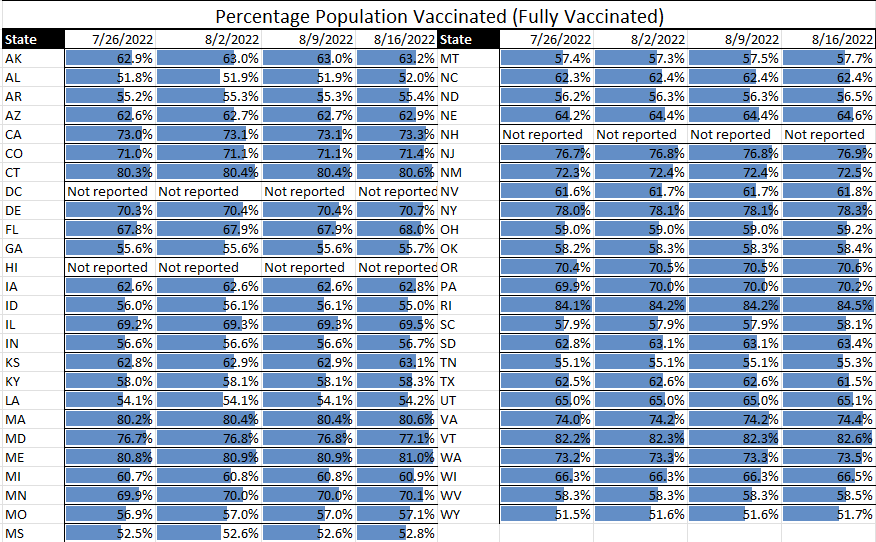
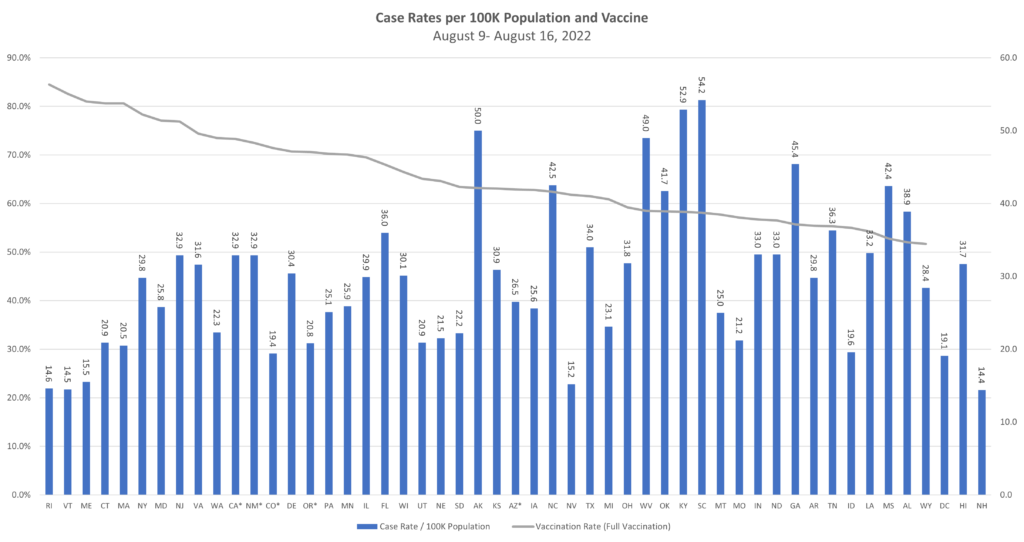
With the COVID-19 pandemic becoming endemic, TAG is expanding its public health offerings to include expanded focus on other workplace communicable diseases. One of the first updates we are making is the inclusion of monkeypox data assessment in our (renamed) 50-State Communicable Diseases Risk Matrix.
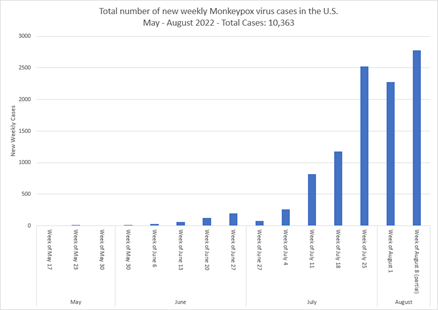
With more than 10,000 monkeypox cases in the U.S. to date, our data (derived from CDC) is showing an increasing slope in newly diagnosed monkeypox virus cases by week since mid-May. Additionally, the greatest numbers of cases are in six states: California, Texas, Illinois, Georgia, Florida, and New York.
Meanwhile, COVID cases continue to decline, showing that the summer surge is clearly on its way down. Although hospital rates remain fairly high in some states, they are a lagging indicator of cases, so we expect these to be declining in the next couple weeks as well. Similarly, while the test positive rate remains high, this can likely be attributed to the reduction in general testing to testing of those who show symptoms, for which we would expect a higher percentage of positives.
In relation to other communicable diseases, such as polio, being detected in the U.S., TAG recommends that businesses encourage employees to keep all vaccinations current. For polio, CDC provides a recommended schedule for routine vaccination, and states that most adults (over 18 years) residing in the United States are presumed to be immune to poliovirus from previous routine childhood immunization and have only a small risk of exposure to poliovirus in the U.S. However, adults who are unvaccinated, incompletely vaccinated, or are at higher risk for exposure to poliovirus, should receive the polio vaccination.
Risk Matrix:
In case you missed it:
- In Tuesday’s Recommendation for Industry, we discussed the “why” behind testing at Day 6 if you’ve been exposed and testing multiple times for COVID if you have symptoms. Read more here.
- CDC unveils streamlined COVID-19 guidance. As was mentioned last week, the expected guidance update was published on Thursday. Key aspects include recommendations to stay up to date with vaccinations. If you’ve been exposed, mask for 10 days and get tested on Day 6. Isolate if you test positive or have symptoms but don’t yet have test results. If positive, stay home and isolate for 5 days; then, if fever-free for 24 hours and symptoms improving, can end isolation. Mask through Day 10. If symptoms worsen after isolation ended, restart at Day 0.
- FDA: Multiple at-home COVID tests needed to confirm negative result. The FDA recommended that people take multiple at-home COVID-19 antigen tests to confirm a negative result, whether they have symptoms or not. The recommendation includes the use of multiple tests over a certain time period, such as 2 to 3 days, then at least one extra test taken 48 hours later to confirm a negative result in people with symptoms. Those without symptoms who think they were exposed should take at least two additional tests, at 48-hour intervals, after a negative result.
- WHO unveils new monkeypox variant names. As the first step in renaming the monkeypox virus and its variants, an expert group convened by the World Health Organization (WHO) agreed on new names for the variants, as the WHO continues collecting proposals on a new name for the disease itself. The experts reached consensus on names for the clades: clade 1 for the former Congo basin (Central African) clade and clade 2 for the former West African clade. The two clade 2 subclades will be known as clade 2a and clade 2b. The clade 2 variants are the main ones circulating in the current global outbreak.
Food Safety & Public Health
- Polio found in NYC wastewater; local circulation likely. Sequencing tests suggest the virus in New York City wastewater is either vaccine-derived poliovirus or variants of the revertant polio Sabin-like type 2 poliovirus, both of which can cause illness in people. CDC stated that there is no cure for polio, but it is preventable through safe and effective vaccination. People most at risk for infection are those who never had the polio vaccine; those who never received all the recommended vaccine doses; and those traveling to areas that could put them at risk for getting polio.
- FDA Releases Paper-Based Versions of the Agricultural Water Assessment Builder Tool. The FDA has released paper-based versions of its agricultural water assessment builder tool in both English and Spanish. The assessment builder was released in March as an online tool to help farms understand the proposed requirements in the Agricultural Water Proposed Rule. The paper-based version is intended to make the content more accessible to a broader array of users. Use is optional.
- FDA – Rumor Control. Earlier this month, FDA published a paper on “Rumor Control.” Stating that “the growing spread of rumors, misinformation and disinformation about science, medicine, and the FDA, is putting patients and consumers at risk,” the paper includes COVID-19 FACTS Q&A. FDA Commissioner Robert Califf has said that combating misinformation is one of his priorities.
- August is National Immunization Month. National Immunization Awareness Month (NIAM) is an annual observance held in August to highlight the importance of vaccination for people of all ages.